Index.
- New Quali-Quantitative formula fields and export to excel.
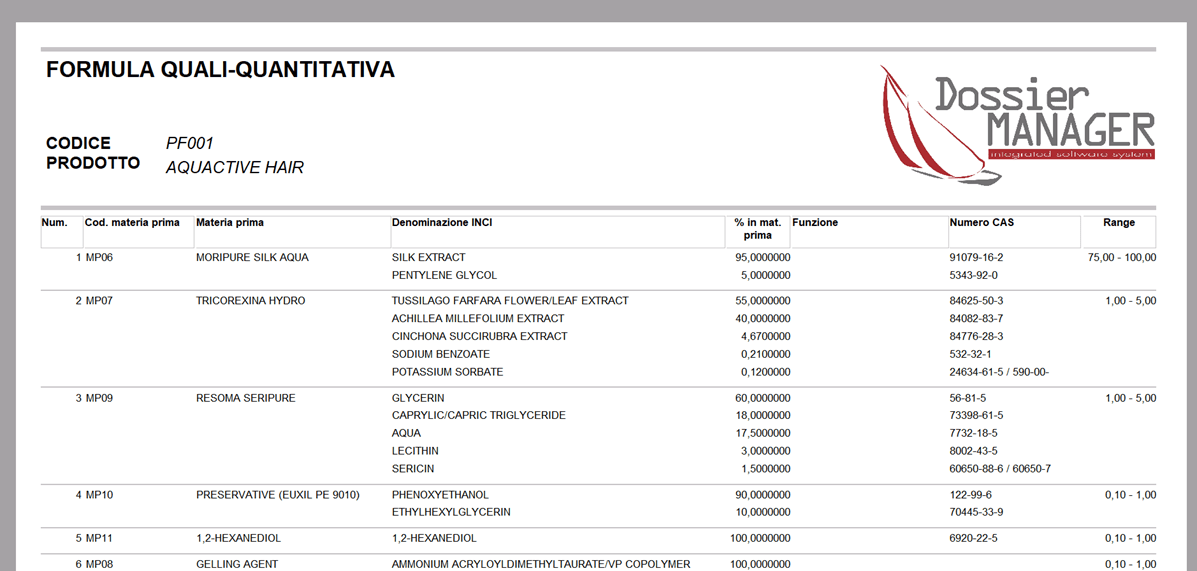
- Range formula.
- Export and print QualiQuanti formula in range.
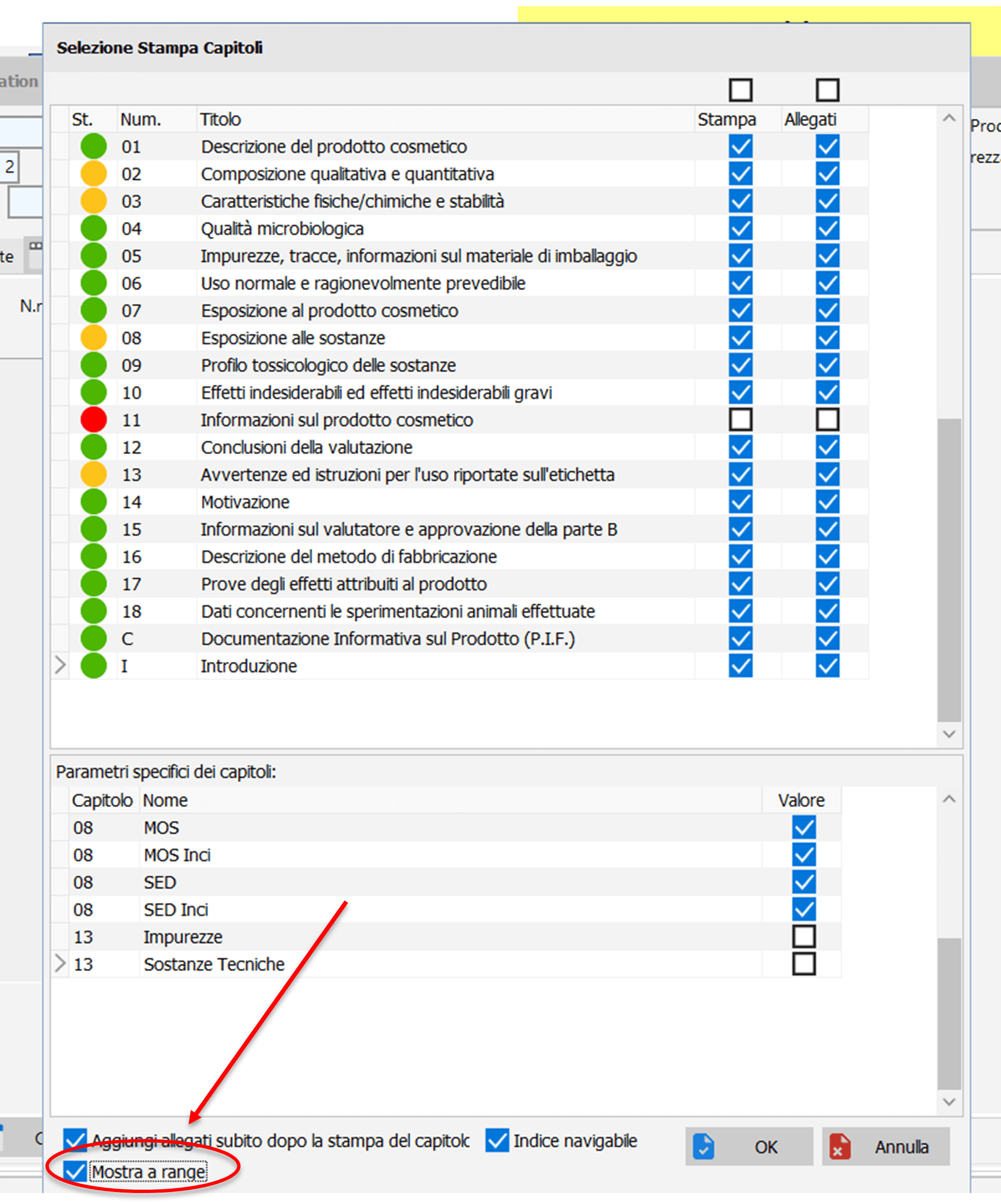
- PIF printing with range formula.
- Advanced PIF management.
- PIF signature.
- PIF index navigability.
- PIF auditing: extended event.
- Advanced PIF versioning management.
- Organization and exclusion of linked documents.
- Advanced PIF structure management.
- Creation of an unprotected version from an already approved pif.
- Black List.
- Query Extractor Module.
- Management of obsolete Quality Control certificates.
- Print file export.
- Advanced specification management.
- Display specifications button.
- Advanced label management.
- Labes Auditing.
New Quali-Quantitative formula fields and export to excel.
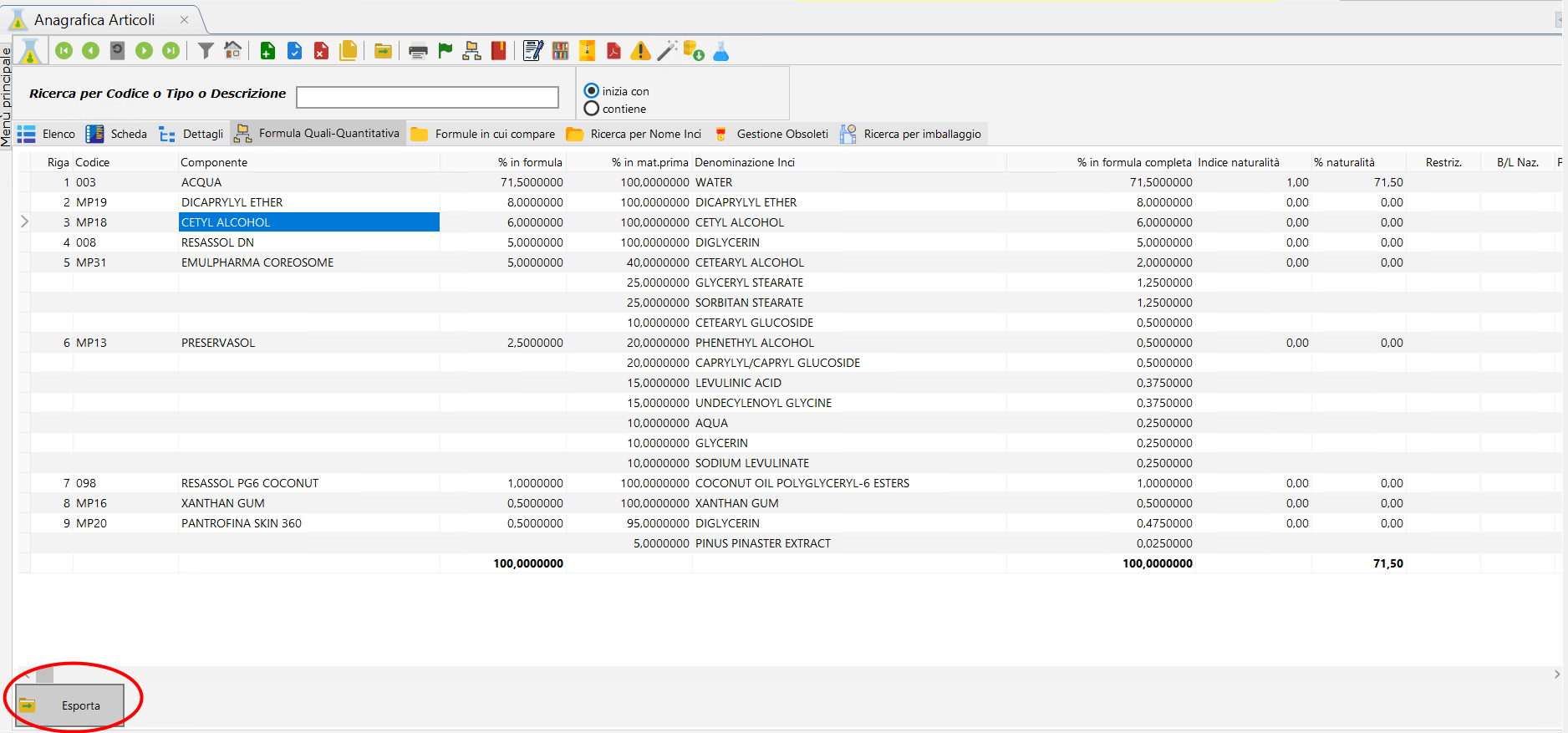
With Dossier Manager Plus you can use new fields in your Quali-Quantitative formula, including the Natural Origin Index em>, % Natural Origin Content, CTFA name, Component Origin, RSPO codeand fields useful for exporting to China such as the NMPA Code, the Trade Name, and the Supplier Name.
The new Quali-Quantitative formula. Click to play.
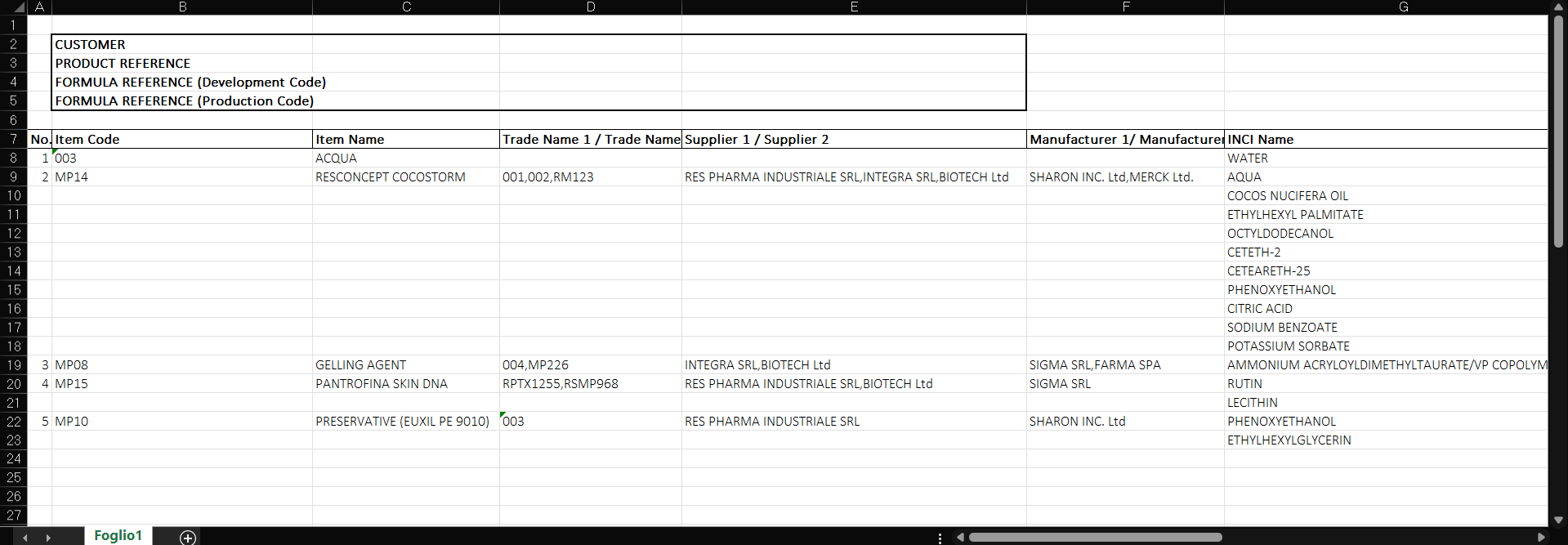
The button to export the QQ formula to Excel format. Click to enlarge.
QQ export in excel format. Click to enlarge.
Range formula.
With Dossier Manager Plus you can expose your formula in range instead of absolute values.
/ Export and print QualiQuanti formula in range.
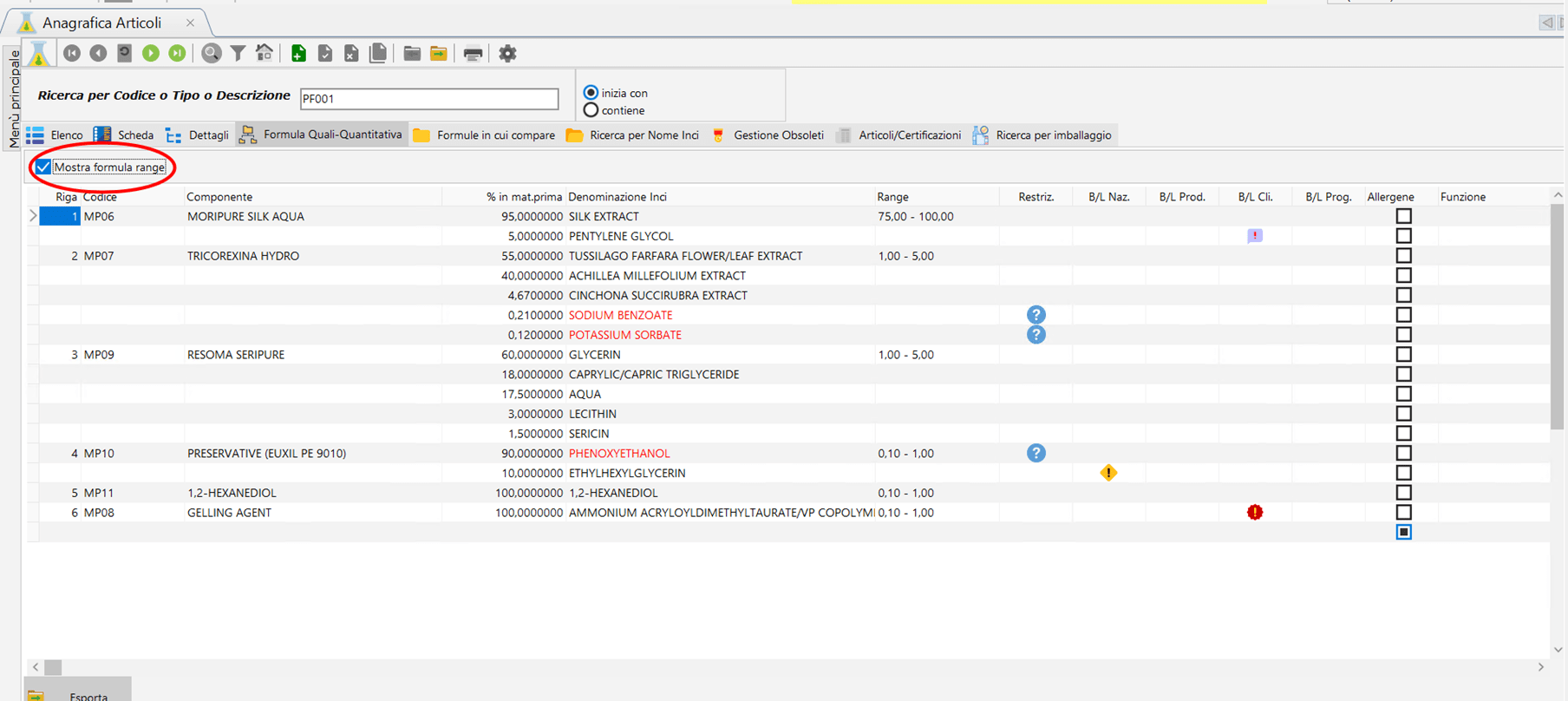
With the “Show range” option button. If selected, the % column in complete formula is shown in range and the % columns in Formula, Naturalness index and % naturalness are hidden. It is possible to export the formula in csv and excel format and print it in pdf format in Italian and in the language of your choice.
QQ formula with range formula. Click to enlarge.
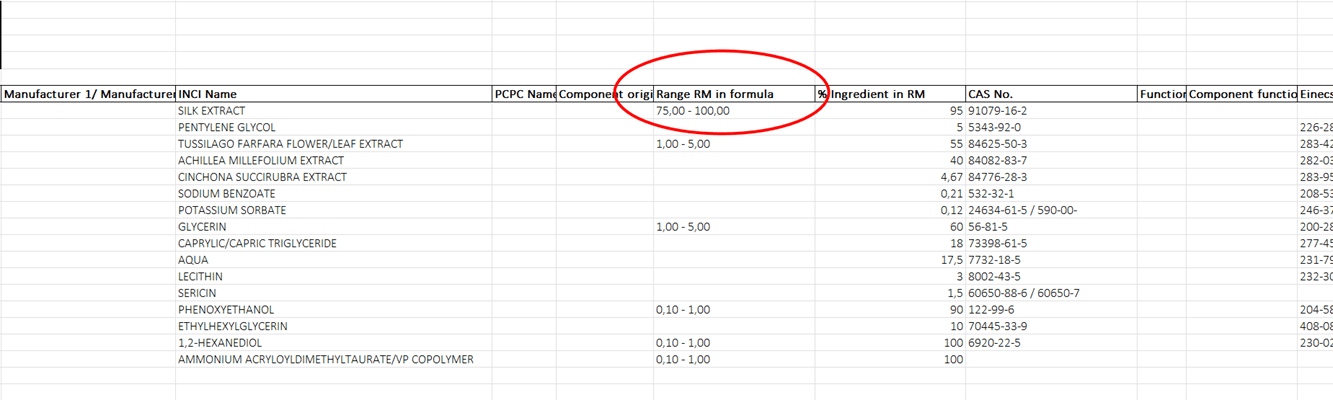
Export with “QQ with range formula” template. Click to enlarge.
The “Quali-Quantitative Formula Range (Italian)” report. Click to enlarge.
/ PIF printing with range formula..
Chapters 2, 8 and 13 can be printed with the option to show the range formula.
Chap. 2: in this case the “% in formula” columns and the “% in formula” column are not printed complete formula” is shown at range.
Chap. 8: in the MOS, MOS-INCI, SED and SED-INCI tables, in place of the “Qty.” column. Perc.” the “Range” column is printed. 100 (pass does not pass) is printed in the calculated value column. The implementation also applies to technical substances and impurities.
Chap. 13: the printing of the column with the range values is independent of the type of label present in the chapter .
Advanced PIF management.
The Dossier Manager Product information File complies with the Colipa 2011 and SANCO 2013 guidelines.
The amount of information and data contained in the document may vary significantly from one user to another, depending on the type of product, the layout of the document, and the internal use of the software.
Dossier Manager Plus allows you to take advantage of a PIF document that is as manageable as possible.
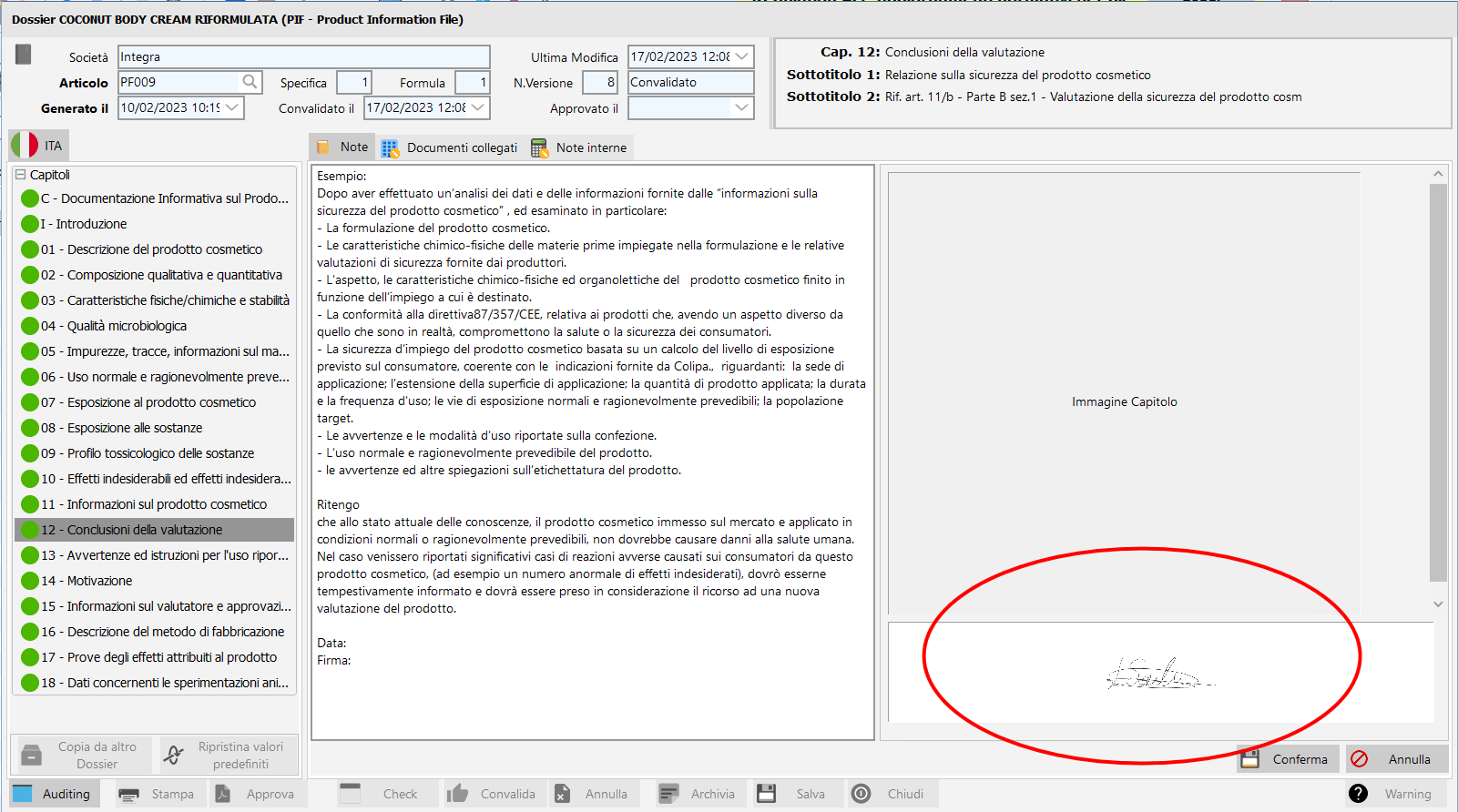
/ PIF signature.
This functionality allows theinsertion of the Security Assessor's signature in chapter 12 of validated BIPs awaiting approval.
The signature of the PIF. Click to play.
The simple electronic signature for the Security Assessor. Click to enlarge.
/ PIF index navigability.
Once you have printed the PIF in .pdf version, you can navigate the file from the index at the end of the document.
The navigable index of the PIF. Click to play.
The navigable index of a PIF. Click to enlarge.
/ PIF Auditing: Extended Event.
This function allows you to record all the changes made to the chapters of the PIF.
PIF Auditing: the Extended Event. Click to play.
/ Advanced PIF versioning management.
Advanced versioning management allows you to modify the PIF generation date during the transition between check and validation.
Includes consistency checks between the date the pif was first generated and the date the specification and formula were created.
The PIF generation date cannot be earlier than the creation of the formula and specification.
If the generation date is changed, the wizard for deleting already validated PIFs is started.
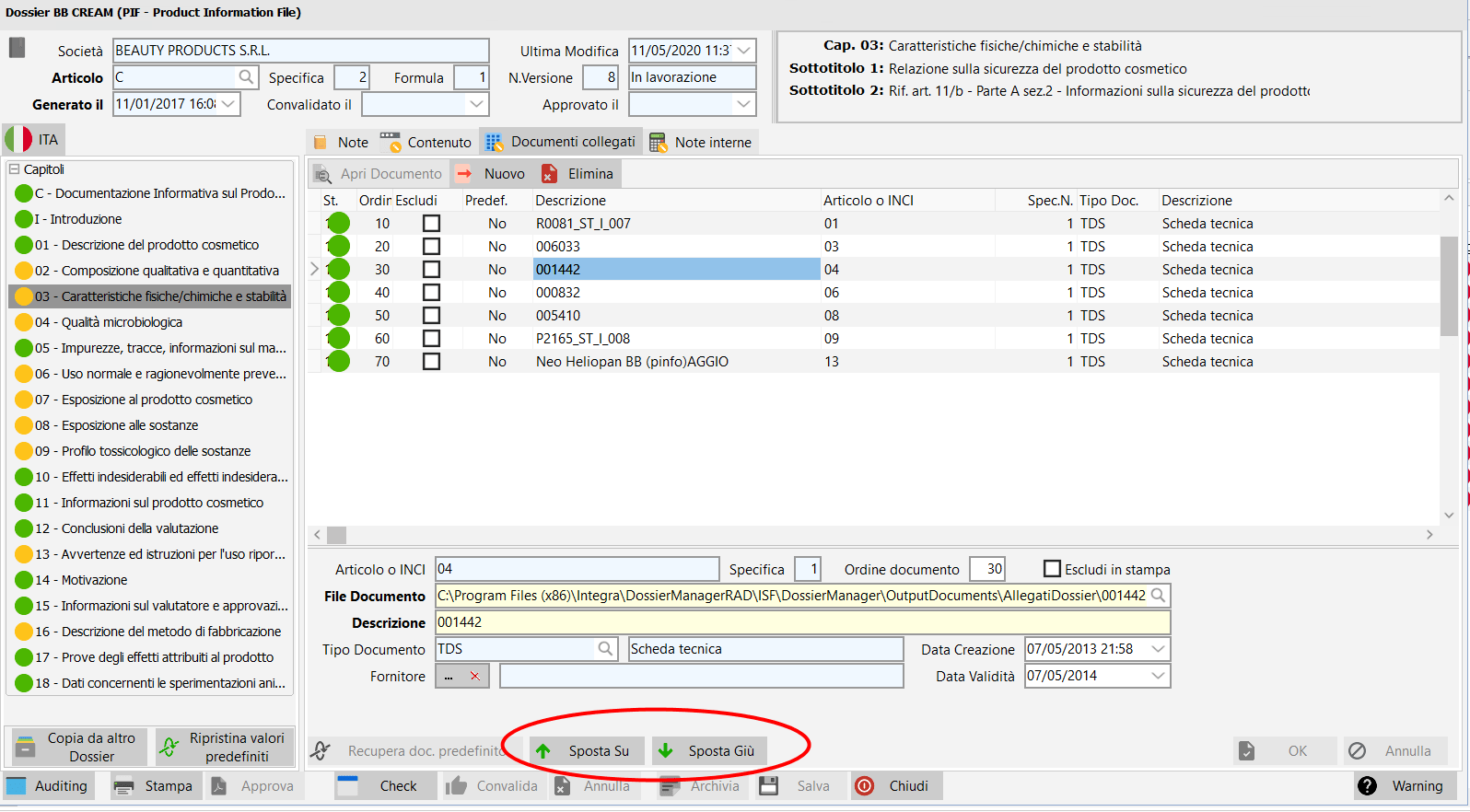
/ Organization and exclusion of related documents.
Within each chapter of the PIF being worked on, it is possible to manage the ordering of the documents in the list, with the move up buttons > and move down to the bottom of the page.
Furthermore, there is the possibility of entering an integer in the "document order" field to establish the order in which you want the documents to appear within the chapter .
You can also select linked documents that you want to exclude from printing.
/ Advanced PIF structure management.
Advanced PIF structure management inserts attachments at the end of each chapter.
/ Creation of an unprotected version from an already approved pif.
With this functionality it is possible to intervene on the generated unprotected PDF to, for example, add signatures to the various pages of the Dossier.
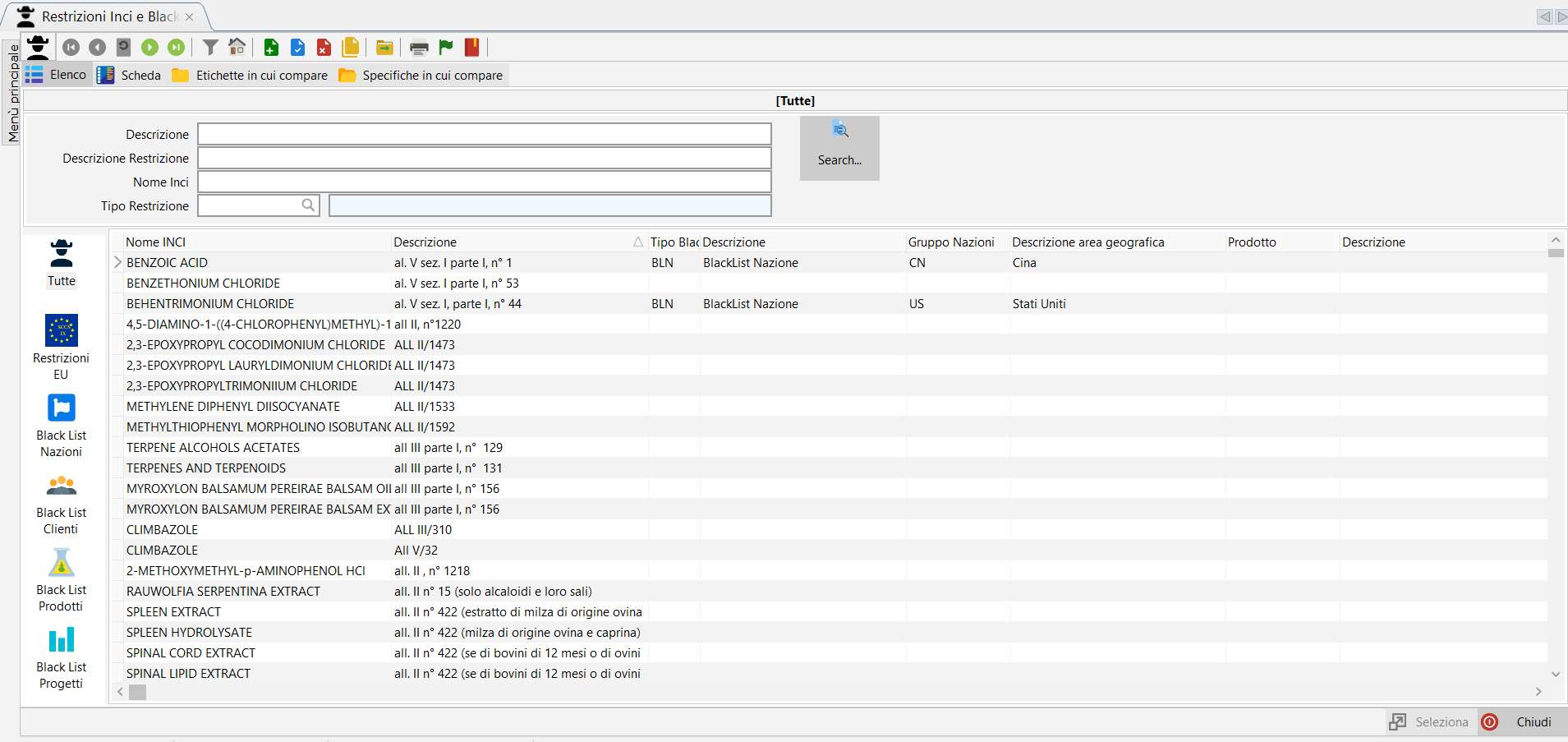
Black List.
Dossier Manager Plus offers blacklist management for those who do not have the module D-Lab.
Black lists allow you to apply different types of restrictions decided internally by the company or by different subjects such as foreign nations, customers or particular suppliers.
How to generate a blacklist from a template. Click to play.
Query Extractor Module.
This feature is particularly useful for System Administrators who must respond to internal requests to process data contained in the Dossier Manager database.
In fact, it allows the user to extract data contained within the Dossier Manager database or in that of DbTransito, using both logical views or predefined stored procedures, or free queries.
The procedure guides the user in defining the extraction mode and any parameters: the result of the extraction is shown in a preview and then saved in an excel file defined by the user. The excel file can also have a template for the definition of the layout.
Each extraction performed is stored in the database with the name of the user, the date and time of extraction and the name of the file produced: each user will see only the list of his own extractions, while an administrator user will see all of them.
The system also allows you to clone a previous extraction to give you the ability to perform the same extraction at later times.
Management of obsolete Quality Control certificates.
Activation of an "archive" button (enabled only on canceled certificates). With the archive command the system will warn you that a PDF copy of the certificate will be created and saved in an archive folder and will delete the record from the database.
Print file export.
The function allows the export of print reports in Word and Excel format in addition to the usual PDF format.
How to export a product label. Click to play.
Gestione avanzata della specifica.
Possibilità, in fase di inserimento, di importare automaticamente i parametri definiti in un modello per tipologia articolo.
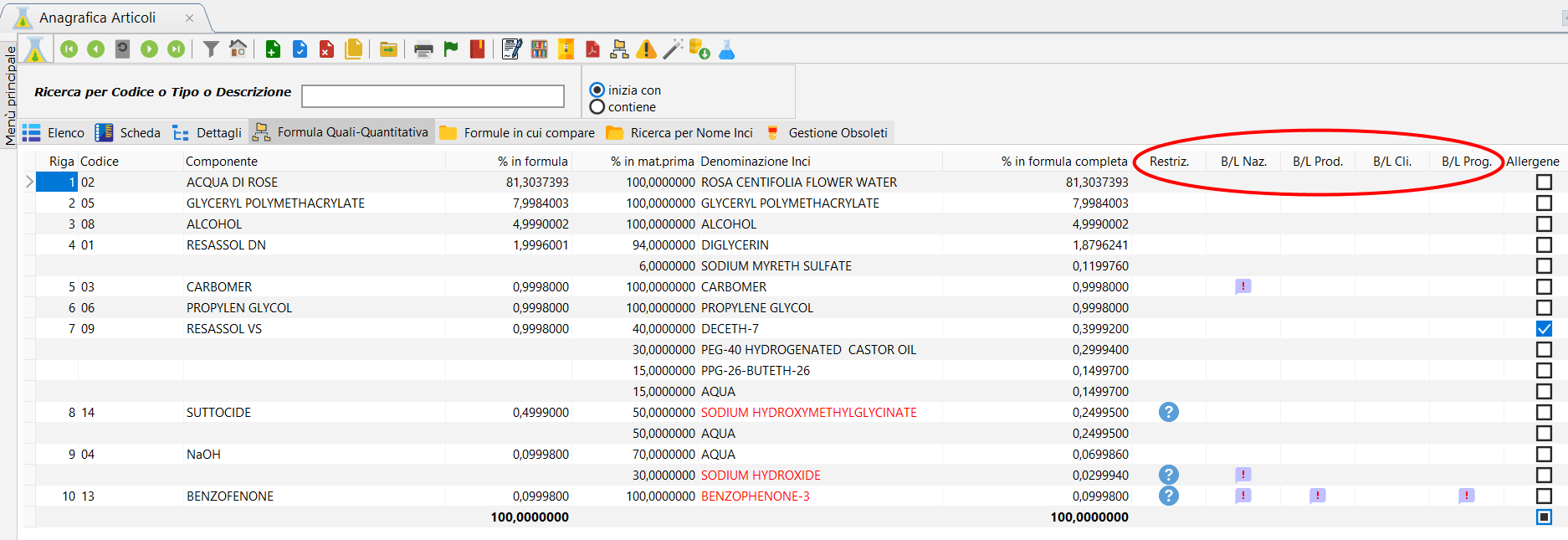
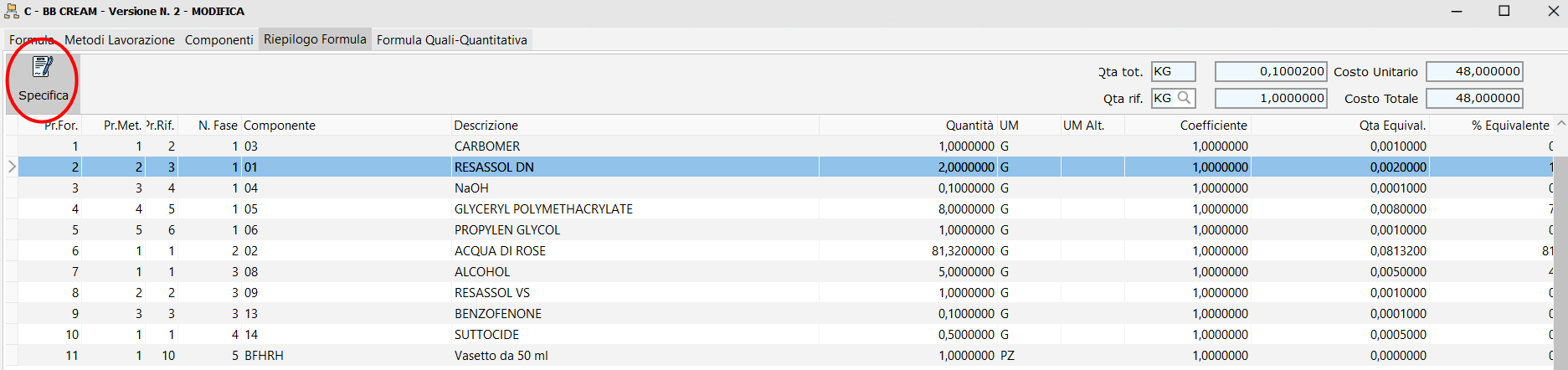
/ Display specifications button.
On the formula management form in the 'Formula Summary' tab, a command button allows you to hook into view the Specification of the component present in the row on which you are positioned. If there is no message, it reports it.
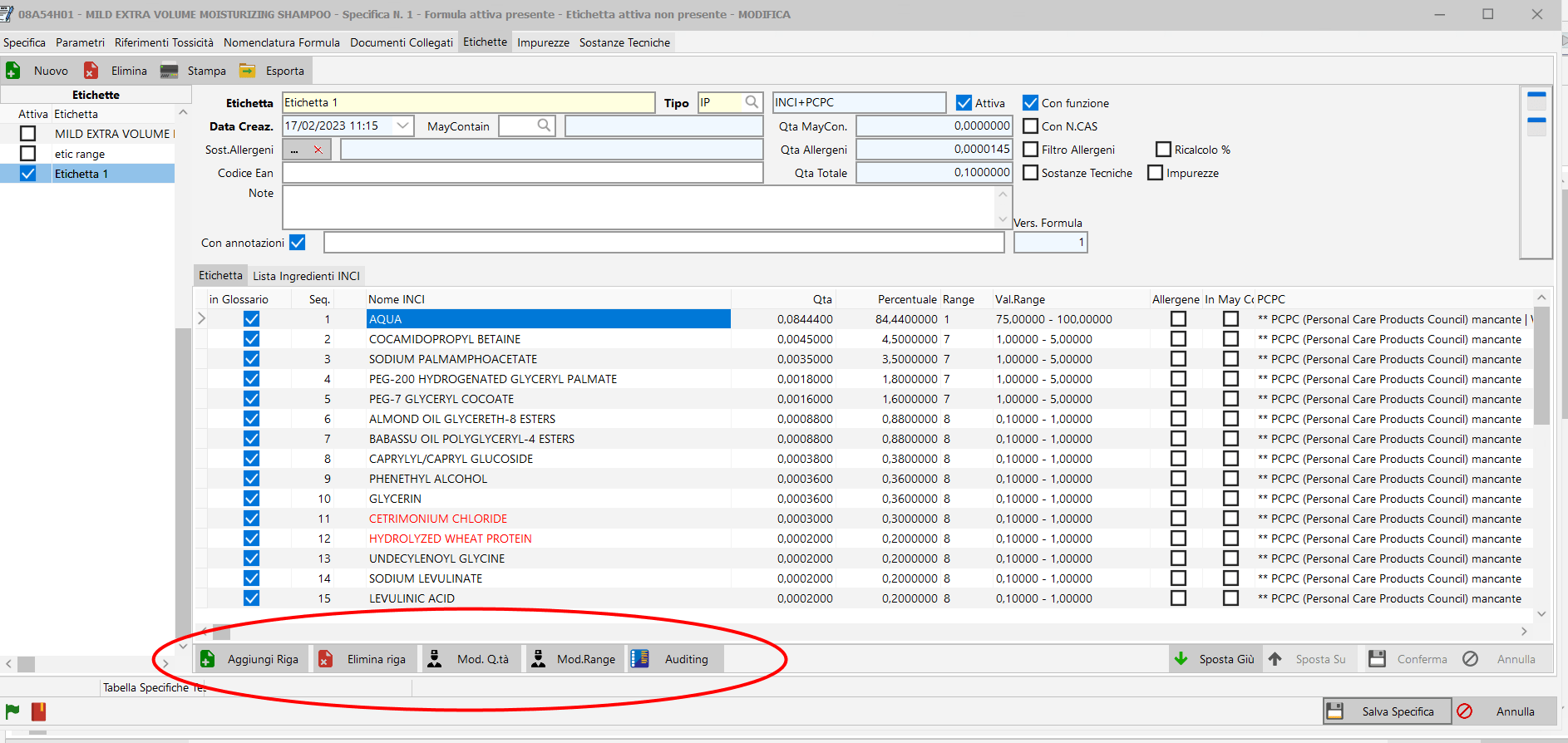
/ Labes Auditing.
The new auditing system allows for easy reporting of all operations carried out on your product labels.
This function also allows you to edit individual entries on the label.