Dossier Manager is the method of management of the Technical-Regulatory Documentation as required by the cosmetic industry from REGULATION (CE) n. 1223/2009.
The aim is to offer an instrument capable of facilitating and optimizing the management of the entire technical area of the company thanks to the affinity of the method to the specific needs of the sector, both regulatory and operational.
The whole method consists of modules dedicated to specific functional areas:
Connect your business.
| PIF Manager | D-Lab | Production | Quality Control | T-LAB | DM Batcher |
|---|---|---|---|---|---|
| Safety Assessor | R&D Manager | Production Manager | Quality Control Manager | R&D Manager | IT Manager |
| Responsabile Regulatory | Marketing Manager | Logistics Manager | Production Manager | ||
| Quality Control Manager | Purchasing Manager | Purchasing Manager |
Data 2 Documents.
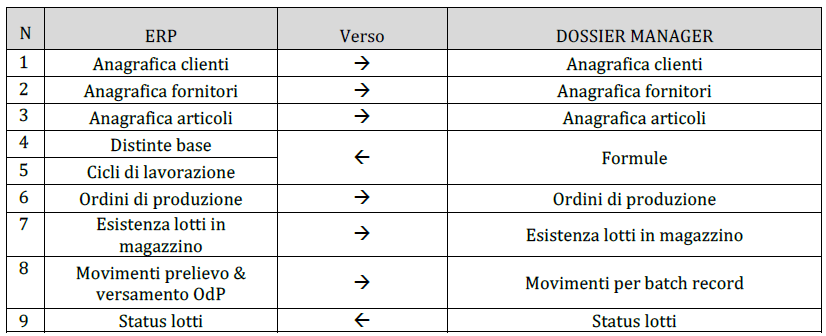
The integration between the modules makes Dossier Manager the ideal tool for managing the technical-productive area of the cosmetic industry. With DM Batcher the modules integrate perfectly into the company structure and are easily interfaced with all types of management that deal with warehouse handling, customer and supplier orders, formulas, technical data and much more.